What are the key differences between standard PCBAs and Medical PCBAs?
Introduction to Medical PCBAs
Printed Circuit Board Assemblies (PCBAs) serve as the backbone of modern electronic devices, facilitating the electrical connection between various components. In the medical field, however, the stakes are significantly higher. Medical PCBAs are tailored for use in healthcare equipment, where precision, reliability, and compliance with stringent safety regulations are paramount. Unlike standard PCBAs, which can tolerate lower levels of performance and reliability, medical PCBAs must meet rigorous standards to ensure they function flawlessly in critical applications.
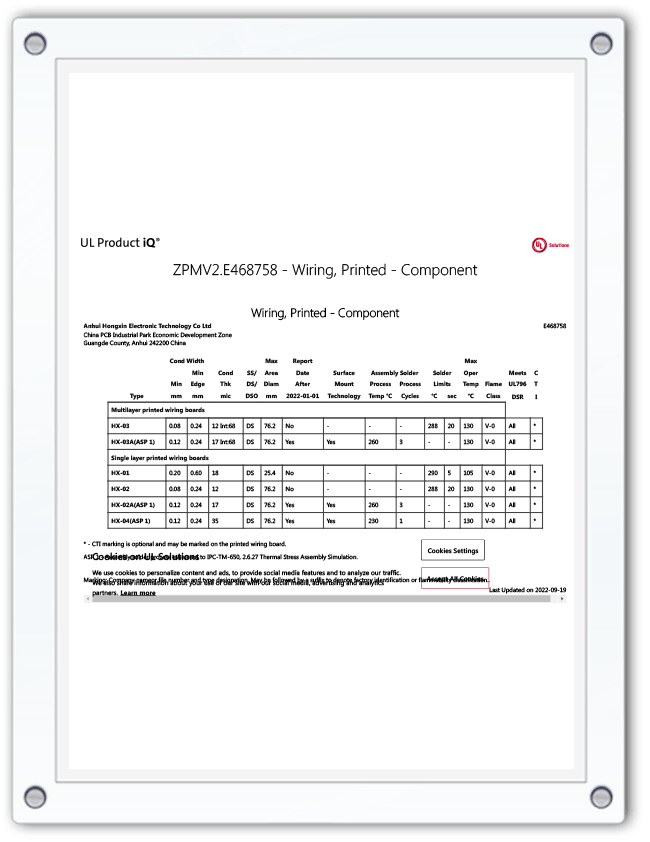
At Anhui Hongxin Electronic Technology Co., Ltd., we specialize in the manufacturing of high-quality PCBAs, including those tailored for medical applications. With advanced production capabilities and certifications from international quality management systems such as ISO9001 and IATF16949, our medical PCBAs are built to meet the most demanding healthcare standards. As a leader in the PCB industry, we understand the importance of producing components that support life-saving equipment with superior performance.
Material and Design Differences
One of the main differences between standard and medical PCBAs lies in the materials and design precision used. Medical PCBAs often require unique substrates and metals that can handle extreme conditions, including exposure to heat, moisture, and mechanical stress. Medical PCBAs frequently use highly thermally conductive metal substrates or biocompatible ceramic substrates. These materials ensure that medical devices can operate under complex in-vivo and out-of-vivo conditions.
In comparison, standard PCBAs typically rely on more cost-effective materials, such as FR-4, which are sufficient for general electronic devices but may not provide the same level of durability or safety required in the medical field. The use of specialized materials for medical PCBAs ensures that they can function in environments that require absolute stability, such as in surgical devices or life-support equipment.
Manufacturing Precision and Process Control
The manufacturing process for medical PCBAs is more precise than for standard PCBAs. Medical devices require micron-level accuracy in their circuit design to support the transmission of critical signals, such as heart rate or blood oxygen levels. This requires medical PCBAs to have multi-layer high-density interconnections and precision down to the millimeter. For instance, Anhui Hongxin Electronic Technology Co., Ltd. utilizes state-of-the-art equipment such as AOI optical inspection machines and peel strength testers to ensure the highest manufacturing standards.
This level of precision is not typically necessary for standard PCBAs, which are designed for less demanding applications. For example, consumer electronics might tolerate a slight error in the design without compromising functionality, while in medical equipment, even the smallest flaw could lead to inaccurate diagnostic results or device malfunction.
Compliance with Medical Standards and Certifications
Another significant difference between standard and medical PCBAs is the strict regulatory compliance that medical devices must adhere to. Medical PCBAs must meet numerous certification standards, such as ISO 13485 for quality management systems in medical devices and FDA approval for medical equipment. These certifications ensure that the components are safe for use in healthcare environments and will not pose any risk to patients or healthcare professionals.
In contrast, standard PCBAs do not face the same level of regulatory scrutiny. While they may need to comply with basic safety and quality standards, they are not subject to the rigorous testing and certification processes that medical PCBAs undergo.
Applications of Medical PCBAs
The applications of medical PCBAs are vast, ranging from home oxygen concentrators and portable monitors to complex diagnostic machines like MRI or CT scanners. These applications demand high-performance circuits that operate with extreme accuracy and reliability. As an example, Anhui Hongxin Electronic Technology Co., Ltd. manufactures PCBAs used in high-end medical imaging devices, where the accurate transmission of signals is essential for clear and precise diagnostics.
In contrast, standard PCBAs are typically used in consumer electronics, automotive applications, or industrial equipment, where the margin for error is less critical. While they may need to handle high voltages or complex data processing, the consequences of a failure are not as immediate or severe as in medical devices.
Conclusion
In conclusion, the key differences between standard PCBAs and medical PCBAs lie in their materials, design precision, manufacturing processes, and regulatory requirements. Medical PCBAs are built to operate with the utmost reliability and precision in environments where human lives are at stake, while standard PCBAs can afford to compromise on these factors to reduce cost. At Anhui Hongxin Electronic Technology Co., Ltd., we are committed to delivering high-quality medical PCBAs that meet the demanding standards of the healthcare industry, ensuring the safety and efficacy of medical devices.
FAQ
What certifications are necessary for medical PCBA?
Medical PCBAs must adhere to certifications such as ISO 13485 for quality management, CE marking, and FDA approval to ensure their safety and reliability in medical devices.
How does the material choice impact the performance of a medical PCBA?
The choice of materials, such as high-thermal conductivity metals or biocompatible ceramics, ensures that medical PCBAs can function effectively under challenging conditions, providing stability in life-critical applications.
Why is precision so important in medical PCBAs?
Medical PCBAs must support micron-level accuracy to ensure the correct transmission of vital signals, such as heart rates or oxygen levels. A small error can lead to inaccurate data, affecting diagnosis and treatment.



 English
English  Español
Español  Français
Français